Starting with 3decision®: exploration of Ikaros protein family – a molecular glue use case
This April 3decision’s Protein of the Month was about the transcription factor Helios for which scientists are developing specific molecular glue. Since molecular glue is a very hot subject in drug design, I decided to elaborate further on this topic using 3decision - a Discngine’s web-based solution that stores and analyzes 3D protein structures. During my onboarding period, I have been using the product, especially the features like basic search, chemistry search and advanced search.
In this blog post, I will take you with me on a step-by-step journey exploring the structural differences of Helios and its family members that aided the design of molecular glue. I will show some of 3decision’s cool features adapted to this specific scientific use case, extracted from this publication “Acute pharmacological degradation of Helios destabilizes regulatory T cells”.
Let’s start with a summary of protein degraders
Small molecule targeted protein degradation (TPD) has recently emerged as a highly promising new modality in drug discovery and has great potential to expand the druggable space. They are mainly sorted into two classes:PROTAC & molecular glue. If you are interested in PROTAC use cases, you can read our Protein of the month from April 2021 or a dedicated use case, both featuring Bromodomain-containing protein 4 (BRD4).
The discovery of the first molecular glue, thalidomide, was serendipitous. A molecular glue is a type of molecule that stabilizes the interaction between two proteins that do not normally interact. The degradation of proteins implicated in disease can be accelerated by molecular glues recruiting cereblon – one of the four proteins constituting the E3 ubiquitin ligase complex. The first complex of thalidomide with cereblon was resolved in 2014. After that, several other structures of cereblon with diverse neosubstrates were published.
“The target of a molecular glue is a single protein, in most degrader cases an E3 ligase. Upon binding, the molecular glue reshapes the protein’s surface inducing the recognition of neosubstrates primarily via novel, non-polar contacts.” (ref here)
Over time, molecular glues became particularly attractive for drug discovery due to their lower molecular weight and more drug-like properties compared to large molecular entities like PROTACs. The C&EN published an article about molecular glue for drug discovery, leading companies in the field and future perspectives.
Molecular glue design
In the abovementioned publication, scientists exploit the TPD approach to redirect an E3 ubiquitin ligase to the transcription factor Helios, an attractive immune-oncology target from the Ikaros zinc finger (IKZF) protein family (read more about the target here). Despite its therapeutical importance, Helios is hard to drug and no Helios-targeting small molecules have been reported so far.
To develop a specific molecular glue for Helios, the discovery project started with already known imide compounds binding to other Ikaros family proteins -i.e. Ikaros and Aiolos-. The IKZF family consists of five members which exhibit specificities at their second zinc finger, as summarized below:
| Protein | Ikaros | Helios | Aiolos | Eos | Pegasus |
|---|---|---|---|---|---|
| Gene | IKZF1 | IKZF2 | IKZF3 | IKZF4 | IKZF5 |
| Second zinc finger difference | Gln146 | His141 | Gln147 | His188 | Arg111 |
| Representative PDB structure | 6H0F | 7LPS | none | 2MA7 | none |
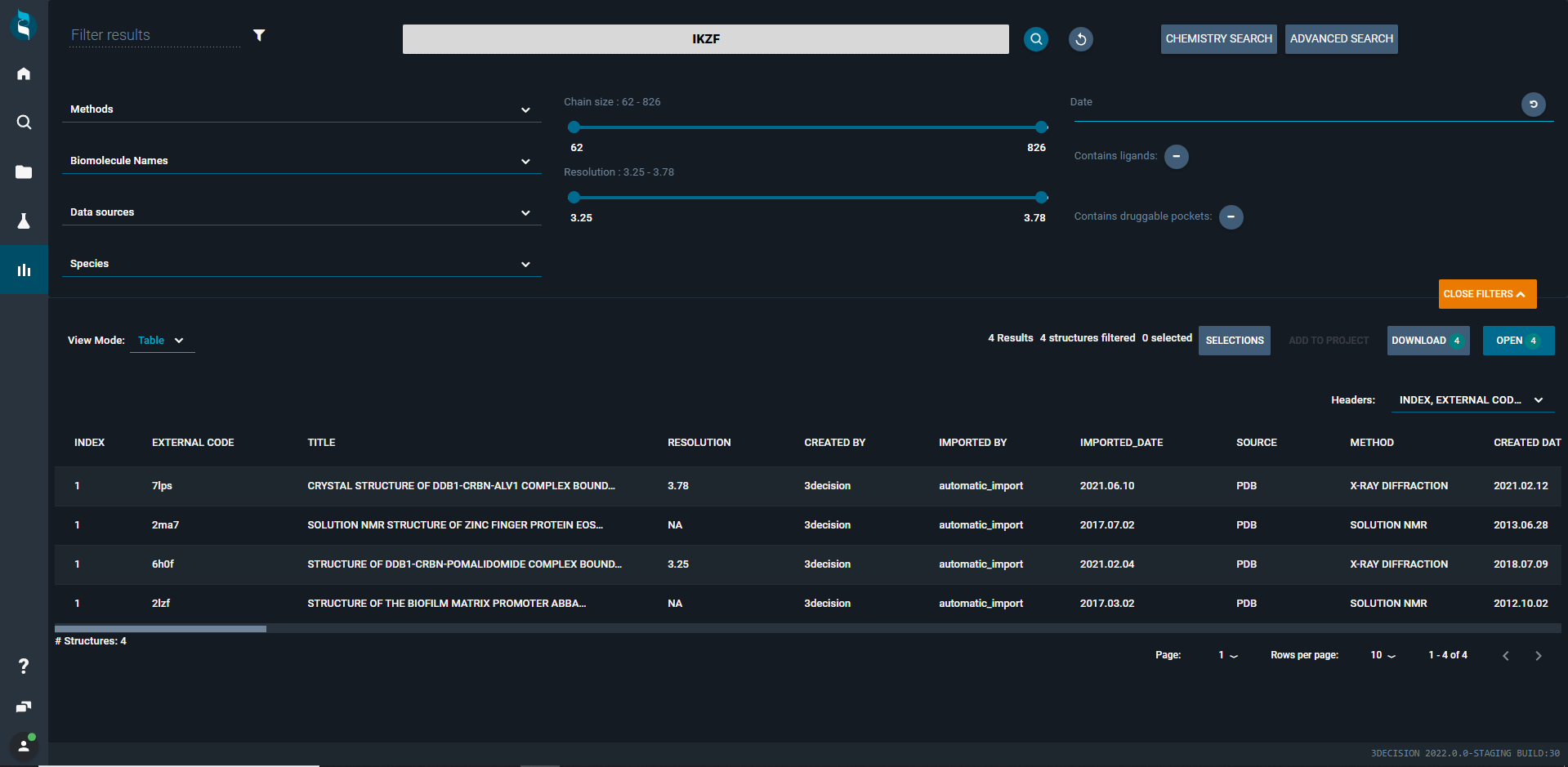
This first step of the project can be done easily in 3decision: Simply type “IKZF” in the 3decision basic search (Home tab). In a matter of seconds, 3decision gives you back a list of four IKZF family protein structures (new Search results tab) publicly available in the PDB (Image 1).
Image 1: Search results for the query based on the protein family name IKZF in 3decision Basic search.
Then, you can refine your search by clicking on “more filters” (orange button). In this new area, you have the possibility to reduce the output list using Methods, Biomolecule Names, Data Sources, Species, Chain size, Resolution, Date, and absence/presence of ligands and druggable pockets (druggability > 0.5).
So, to keep only human structures, just select Human in the Species dropdown menu. It ends up with 3 structures. You can also change the View Mode from Structure to Ligand, Target or Table depending on what you are looking for (click on the images below to zoom in).
Structure View Mode
Ligand View Mode
Target View Mode
Table View Mode
Looking back into the article, I found out that scientists performed different mutations on the Ikaros protein, replacing the key difference residues Glutamine 146 with Histidine (residue specific to Helios) and observed reduced imide-induced cereblon binding. On contrary however , by mutating Glutamine 146 into Alanine, they observed a similar cereblon binding. These facts permit us to understand that it is histidine steric/electronic hindrance and not loss of interactions with glutamine that is responsible for the decrease in cereblon binding affinity. Based on those key insights, scientists synthesized more flexible compounds by modifying CRBN binding core.
They tried a more flexible CRBN binding core than isoindolinone, an anilinomaleimide core, and saw that this compound is able to engage CRBN in cells. Then, they screened a small, focused library of anilinomaleimides using a CRBN-Helios time-resolved fluorescence energy transfer (TR-FRET) dimerization assay. After several rounds of medicinal chemistry optimization, they discovered ALV1 and ALV2 (Image 2).
These compounds have distinct pharmacological degradation profiles: ALV1 degrades both Ikaros and Helios, and ALV2 is more selective for Helios.
Image 2: The discovered molecular glue compounds - ALV1 and ALV2 - after several Design-Make-Test-Analyze cycles done by the team
Let’s visualize where this key residue is located. For this step, just select the Ikaros 6H0F structure from the Search results and opened it in Workspace tab (Image 2).
Image 3: Select desired structure for visualization in a click .
The focus is automatically done on the pocket containing pomalidomide. Indeed, in 3decision, the reference pocket will be the one with the best druggability score (ref here).
Ligand coloring
The proteins are shown as cartoons and the residues lining the pocket as lines. The color of each chain is the same on the left and the right part. In the 3D Viewer, when you click on the three horizontal lines –ie. hamburger button (Objects panel button), you can Show, Hide, Center and Color the different loaded objects: Ligand, Pocket, Cartoon, Water molecules, Surface, Contacts and Measurements. Atom numbers are appearing when you hover over with a mouse, facilitating the identification of Glutamine 146.
Superposition of structures has never been easier
Then, to compare cereblon-Ikaros structure - 6H0F - to cereblon-Helios structure – 7LPS – go back on the results tabulation, your workspace won’t be cleared. From the search previously done, select the structure 7LPS and add it to the existing Workspace. 3decision® automatically superposes the two protein structures based on the selected pockets. The structure 7LPS contains one part of E3 ubiquitin ligase – DDB1 and Cereblon proteins - in complex with the compound ALV1.
Using the Highlight Mode – Pocket, you can easily localize the pocket specificities between Ikaros and Helios (Image 4).
Image 4: The comparison of cereblon-Ikaros structure (6H0F) and cereblon-Helios structure (7LPS ). Superposition in 3decision is based on the selected pockets. The Highlight mode shows the key difference in sequence - Glycin 146 in Ikaros (in pink ) and Histidine141 in Helios (in blue).
How does superposition feature work in 3decision?
-
By default for each structure, the reference pocket is the one with the bigger druggability score and/or in complex with the biggest ligand.
-
If the two structures are containing the same biomolecule, i.e protein, the binding site residues are selected on both structures and a distance weighted optimization is run to superpose the two structures.
-
If the two structures are not containing the same biomolecule, the first step is to identify the best matching pocket. To do that, a sequence alignment of all biomolecules composing the reference pocket versus all biomolecules in the structure to superpose is run. The best matching alignment is kept. Then on this identified biomolecule the pocket overlapping the most with the pocket aligned on the reference structure is selected. From this, the residue mapping allowing the distance weighted superimposition is extracted.
-
If these two possibilities don’t work (no homology), no superposition is possible but you can choose to do a manual superimposition (selecting the two corresponding chains to superpose)
Now let's go a step further and see how the designed molecule ALV1 performs with the off-target GSPT1. GSPT1 is a regulator of translation, and its degradation leads to high cytotoxicity. Fortunately, ALV1 was shown to not induce GSPT1 degradation. In order to understand why, add one interesting structure containing cereblon in complex with GSPT1, the structure 5HXB for example. It contains 3 biomolecule chains: CRBN_HUMAN, ERF3A_HUMAN and DDB1_HUMAN. As our reference pocket is at the interface between CRBN_HUMAN and IKZF1_HUMAN, the best alignment with 6H0F will use CRBN_HUMAN biomolecule.
You can directly add 5HXB from the workspace by clicking on “add structures”, your workspace won’t be cleared. Just type the code 5HXB, create a collection GSPT1 and add the structure to the existing Workspace. Click on the eye button, et voilà! No need to identify the chains to be superposed,all is done as if by magic! It recognizes a common biomolecule lining the binding site: CRBN_HUMAN and just did a distance weighted superimposition.
Now keep only the ligand of Helios (7LPS) and GSPT1 (5HXB) and hide GSPT1 ligand. You can see that ALV1 clashes with Valine 536 because of the common 3-chloro-4-methylphenyl tail and Glutamine 534 because of its hydrophobic ethylbenzene moiety. You can measure distances to verify, click on the ruler and select the two atoms of interest. Both residues are at less than 2 angstroms from ALV1. These structural insights can explain why ALV1 is not able to induce GSPT1 degradation (Image 5).
Image 5: Superposition of Helios with ALV1 and off-target GSPT1 which shows key residues that hinder the binding of ALV1 to the off-target and thus avoid side effects.
Conclusion
I showed you some basic features from 3decision: collecting, filtering, superposing and comparing structural data. In my opinion, the nicest ability of 3decision is the superposition without any effort. In the field of molecular glue, where cereblon is the common protein between two PDB structures, it facilitates the analysis to provide a structural rationale for different behaviors regarding neosubstrates - .i.e Ikaros, Helios, and GSTP1.
3decision® is now available on AWS. Discover the five advantages of 3decision AWS for pharma and biotech in this article written by our cloud expert Alexandre Gillet.